- Monocytes-Inflammation-NGF Group
(Coordinators: Véronique Freund-Michel (Full Professor), Clément Bouchet (CDD-LRU); Participants: Arnaud Maurac (Hospital Practitioner)) - Mechanosensitive Channels and Particulate Pollutants Group
(Coordinators: Isabelle Baudrimont (Full Professor), Thomas Ducret (Full Professor), Jean‑François Quignard (Full Professor)) - Technical staff: Marilyne Campagnac (TCH), Paul Robillard (TCH), Benjamin Péré (TCH)
Pulmonary hypertension (PH), defined as an elevation of mean pulmonary arterial pressure above 20 mmHg, is a common cardiovascular comorbidity of chronic obstructive pulmonary disease (COPD). PH associated with COPD (PH-COPD) significantly increases medical costs and decreases 5-year survival of patients compared to COPD patients without PH or those with other forms of PH. The underlying pathophysiological mechanisms of PH-COPD remain unclear, and no targeted therapies are currently available for these patients.
Moreover, human exposure to airborne particulate matter is a major public health concern. Numerous epidemiological studies have identified particulate matter (PM) and nanoparticles (NPs) as contributors to the onset and exacerbation of respiratory diseases such as COPD, as well as of cardiovascular diseases including PH and heart failure. Patients with pre-existing pulmonary vascular diseases like PH-COPD may therefore be particularly vulnerable to these environmental exposures.
Our main objectives are to elucidate the pathophysiological mechanisms underlying PH-COPD and identify new potential therapeutic targets. Given the poor prognosis of PH-COPD patients and the limited number of studies specifically addressing this condition, PH-COPD constitutes a major public health challenge. Identifying new therapeutic targets may pave the way for innovative treatments and improve patient outcomes. Additionally, we aim to evaluate
Recent Research
Monocytes-Inflammation-NGF Group
Our previous work demonstrated increased pulmonary expression of nerve growth factor (NGF) in PH‑COPD patients compared to controls, and revealed a role for NGF in vascular remodelling, inflammation, and altered pulmonary arterial reactivity (Freund-Michel et al, 2015).
- Recent studies identified mechanisms through which NGF promotes pulmonary arterial hyperreactivity via activation of its TrkA receptor on pulmonary arterial smooth muscle cells. In these cells, NGF chronically regulates Connexin-43 (Cx43) expression and function (Cardouat et al, 2024), and acutely increases intracellular calcium concentrations while enhancing calcium sensitivity of the contractile machinery (Bouchet et al, in submission).
- We then showed that inflammation contributes to NGF increased levels in PH (Bouchet et al, 2022). Our current studies are now exploring interactions between NGF and monocytes, key inflammatory cells involved in both PH and COPD. We have shown that NGF stimulates monocyte release of pro-inflammatory cytokines and factors involved in pulmonary arterial remodelling (Verres et al, 2024).
- In collaboration with the COPD group (Team 2 of our Laboratory), we helped to develop and characterize a novel model of early COPD, where we identified a role for the CXCL12-CXCR4 axis in right ventricular remodelling (Dupin et al, 2025).
Mechanosensitive Channels and Particulate Pollutants Group
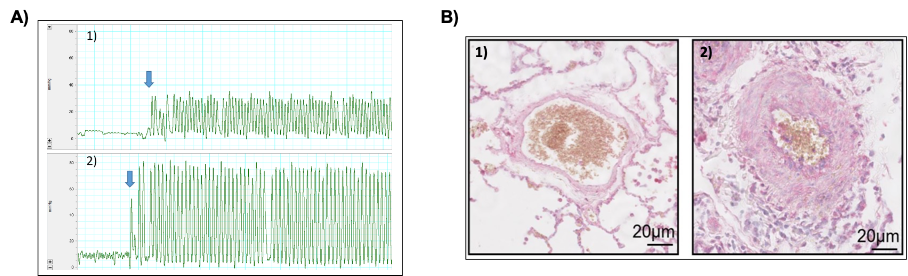
Our previous work demonstrated disrupted intracellular calcium homeostasis in PH. Specifically, we identified a role for mechanosensitive ion channels of the TRP and Piezo families (notably TRPV4 and Piezo1) in PH pathogenesis. These channels transduce physical stimuli (blood pressure, shear stress, matrix stiffness) into calcium signalling, thus contributing to cellular responses and calcium regulation.
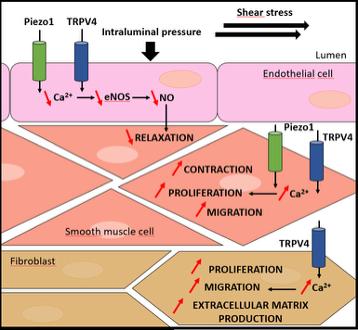
(Adapted from Barbeau et al., Rev Mal Respir, 2022)
- We have shown that the expression of TRPV4 and Piezo1 channels is altered during PH, both in smooth muscle cells, endothelial cells, and adventitial fibroblasts. These mechanosensitive channels are involved in key pathophysiological processes in PH, including vascular tone regulation, cell proliferation/migration, and inflammation. As such, they represent potential therapeutic targets in PH (Barbeau et al, 2021).
- In collaboration with Dr. Percherancier (University of Bordeaux), we developed novel biosensors compatible with bioluminescence resonance energy transfer (BRET) technology: intermolecular biosensors to assess protein-protein interactions and channel relocalization, and intramolecular biosensors to investigate conformational changes and ion flux within the nanodomain of the channel pore (Chappe et al, 2021; Chappe et al, 2022). Using these tools, we demonstrated that hypoxia modulates the expression of TRPV4 at the plasma membrane, and that this membrane localization is influenced by cell confluence (Barbeau et al, 2022).
- In parallel, we showed that carbon black nanoparticles (FW2) and nickel oxide nanoparticles (NiO NPs) induce oxidative stress and an adaptive cellular response in human pulmonary artery endothelial cells (HPAECs), leading to disrupted intracellular calcium homeostasis (via altered TRPV4 channel activity), mitochondrial dysfunction, and apoptosis (Deweirdt et al, 2020; Germande et al, 2022a) (in collaboration with Prof. Baeza, University Paris Diderot, and Prof. Baudrimont, University of Bordeaux). We also demonstrated that these effects are exacerbated under pathological flow conditions mimicking the hemodynamic environment of PH, suggesting that exposure to FW2 and NiO NPs could worsen disease-related vascular alterations in PH patients (Germande et al, 2022b; Deweirdt et al, 2022).
- Finally, an innovative study revealed that the intrinsic oxidative potential of PM2.5 may serve as a predictive marker of particulate toxicity. Moreover, these fine particles promote excessive production of reactive oxygen species (ROS), resulting in redox imbalance, reduced nitric oxide (NO) bioavailability, and disturbed calcium homeostasis, all of which may contribute to endothelial dysfunction (Crobeddu et al, 2020) (collaboration with Prof. Baeza, University Paris Diderot).
Ongoing projects
Monocytes-Inflammation-NGF Group
- Role of Interleukin-1β in the Pathophysiological Effects of NGF in Pulmonary Hypertension
Principal Investigator: Véronique Freund-Michel
In collaboration with Prof. Katia Boniface (ImmunoConcept Laboratory, CNRS UMR5164, Bordeaux) - Characterization of Circulating Monocytes in Patients with COPD-Associated Pulmonary Hypertension (PH-COPD)
Principal Investigator: Clément Bouchet
In collaboration with Prof. Maéva Zysman (COPD Group, Team 2 of our Laboratory, and coordinator of the COBRA cohort including COPD patients with or without PH) - Role of the Inflammatory NGF–TrkA–Monocyte Axis in the Development of PH and Associated Right Heart Failure in COPD Patients
Principal Investigator: Véronique Freund-Michel
In collaboration with Prof. Maéva Zysman (COPD Group, Team 2 of our Laboratory, and coordinator of the COBRA cohort) and Prof. Jean Guillon (BALI Team, ARNA Laboratory, INSERM U1212 – CNRS UMR 5320, Bordeaux) - Role of the miR-147-BDNF Interaction in Vascular Pathologies
Principal Investigator: Véronique Freund-Michel
In collaboration with Prof. Andreas Schober (Vascular Medicine Group, Institute for Cardiovascular Prevention, University Hospital of Ludwig-Maximilians-Universität Munich – LMU Munich, Germany) - Identification of Blood Biomarkers to Distinguish Patients with COPD from Those with PH-COPD
Principal Investigator: Clément Bouchet
In collaboration with Prof. Maéva Zysman (COPD Group, Team 2 of our Laboratory, and coordinator of the COBRA cohort), Prof. David Montani, and Prof. Laurent Savale (INSERM UMR-S999, Le Plessis-Robinson)
Mechanosensitive Channels and Particulate Pollutants Group
- Characterization of Mechanosensitive Channels Using Bioluminescence Resonance Energy Transfer (BRET)
Principal Investigator: Thomas Ducret
In collaboration with Dr. Yann Percherancier (Laboratory for Integration of Material to the System – IMS, CNRS UMR5218, University of Bordeaux) - Identification of Novel Natural Compounds from Brazilian Plant Biodiversity Targeting Mechanosensitive Ion Channels for the Treatment of PH
Principal Investigator: Jean-François Quignard
In collaboration with Prof. Isaac Medeiros (Department of Pharmacology, Federal University of Paraíba, João Pessoa, Brazil) - Matrix Stiffness and Mechanosensitive Channel Piezo1
Principal Investigator: Jean-François Quignard
In collaboration with Dr. Alexandra Gaubert (ARNA Laboratory – INSERM U1212 / CNRS UMR 5320, ChemBioPharm Team, University of Bordeaux) - Impact of Various Types of Nanoparticles on Pulmonary Arterial Reactivity
Principal Investigator: Isabelle Baudrimont
Fundings
Monocytes-Inflammation-NGF Group
- “Danièle Hermann – Women’s Heart” Research Program, Cardiovascular Research Foundation, Institut de France – Véronique Freund-Michel
- “Research, Training, or Evaluation” Program, Fund for Chronic Diseases Requiring Medical-Technical Support, Bordeaux University Foundation – Véronique Freund-Michel
- “Emergency Cardiovascular Pathophysiology Research” Program, Fondation pour la Recherche Médicale (FRM) – Véronique Freund-Michel
- FRM Doctoral Fellowship – “Jeanne-Philippe Béziat 2020 Award” for PhD Funding (for C. Bouchet) – Véronique Freund-Michel
- Final-Year PhD Fellowship, Cardiovascular Research Working Group (GRRC), a Division of the French Society of Cardiology (SFC) (for C. Bouchet) – Véronique Freund-Michel
- “Platforms and Research in Respiratory Health” Grant, Fondation du Souffle – Christelle Guibert / Véronique Freund-Michel
Mechanosensitive Channels and Particulate Pollutants Group
- Collaborative Research Project – Industry Partnership (ANR-PRCE), French National Research Agency – “CANALBRET” – Thomas Ducret / Jean-François Quignard
- Nouvelle-Aquitaine Region – Higher Education and Research Program (ESR) – “PHYSTRIG” – Thomas Ducret / Jean-François Quignard
- Mobility Grant, STS Department, University of Bordeaux (Prof. Isaac Medeiros) – Jean‑François Quignard
- Sandwich PhD Fellowship (for J. De Souza Junior), Federal University of Paraíba, Brazil – Jean-François Quignard
- Doctoral Fellowship – “Mariane Josso Award”, Fondation pour la Recherche Médicale (FRM) (for J. Deweirdt) – Isabelle Baudrimont
- ANR-PRC “CYTOTOX” Project, French National Research Agency – Isabelle Baudrimont
Main bibliography
- Dupin I, Henrot P, Maurat E, Abohalaka R, Chaigne S, El Hamrani D, Eyraud E, Prevel R, Esteves P, Campagnac M, Dubreuil M, Cardouat G, Bouchet C, Ousova O, Dupuy JW, Trian T, Thumerel M, Bégueret H, Girodet PO, Marthan M, Zysman M, Freund-Michel V, Berger P. Blocking CXCR4 improves pulmonary and cardiac outcomes in a mouse model of early COPD. American Journal of Respiratory Cell and Molecular biology 2025 ; Online ahead of print.
- Cardouat G, Douard M, Bouchet C, Roubenne L, Kmecová Z, Esteves P, Brette F, Guignabert C, Tu L, Campagnac M, Robillard P, Coste F, Delcambre F, Thumerel M, Begueret H, Maurac A, Belaroussi Y, Klimas J, Ducret T, Quignard JF, Vacher P, Baudrimont I, Marthan R, Berger P, Guibert C#, Freund Michel V#. NGF increases Connexin-43 expression and function in pulmonary arterial smooth muscle cells to induce pulmonary artery hyperreactivity. Biomedicine & Pharmacotherapy 2024 ; 174 : 116552. #equal contribution as last authors.
- Verres Y, Bodin A, Chevret S, Victoni T, Gicquel T, Barreto E, Freund-Michel V, Lagente V. Effects of the nerve growth factor and its carrier protein on the inflammatory response from human monocytes. Fundamental and Clinical Pharmacology 2024 ; 38 : 940.
- Barbeau S, Porto Ribeiro T, Gilbert G, Cardouat G, Baudrimont I, Freund-Michel V, Guibert C, Marthan R, Vacher P, Quignard J-F, Ducret T. Involvement of the Piezo1 and TRPV4 stretch-activated channels in pulmonary hypertension. Revue des Maladies Respiratoires 2022 ; 39 : 79.
- Barbeau S, Joushomme A, Chappe Y, Cardouat G, Baudrimont I, Freund-Michel V, Guibert C, Marthan R, Berger P, Vacher P, Percherancier Y, Quignard J-F, Ducret T. Cell Confluence Modulates TRPV4 Channel Activity in Response to Hypoxia. Biomolecules 2022 ; 12 : 954.
- Bouchet C, Cardouat G, Douard M, Coste F, Robillard P, Delcambre F, Ducret T, Quignard JF, Vacher P, Baudrimont I, Marthan R, Berger P, Guibert C#, Freund-Michel V#. Inflammation and Oxidative Stress Induce NGF Secretion by Pulmonary Arterial Cells through a TGF-β-Dependent Mechanism. Cells 2022 ; 11 : 2795. #equal contribution as last authors.
- Chappe YL, Pierredon S, Joushomme A, Molle P, Garenne A, Canovi A, Barbeau S, Poulletier De Gannes F, Hurtier A, Lagroye I, Ducret T, Quignard J-F, Compan V, Percherancier Y. Genetically-encoded BRET probes shed light on ligand bias-induced variable ion selectivity in TRPV1 and P2X5/7. Proceedings of the National Academy of Sciences of the United States of America (PNAS) 2022 ; 119 : e2205207119.
- Deweirdt J, Ducret T, Quignard J-F, Freund-Michel V, Lacomme S, Gontier E, Muller B, Marthan R, Guibert C, Baudrimont I. Effects of FW2 Nanoparticles Toxicity in a New In Vitro Pulmonary Vascular Cells Model Mimicking Endothelial Dysfunction, Cardiovascular Toxicology 2022 ; 22 : 14.
- Germande O, Baudrimont M, Beaufils F, Freund-Michel V, Ducret T, Quignard J-F, Marie-Hélène E, Lacomme S, Gontier E, Mornet S, Bejko M, Muller B, Marthan R, Guibert C, Deweirdt J# Baudrimont I#. NiONPs-induced alteration in calcium signaling and mitochondrial function in pulmonary artery endothelial cells involves oxidative stress and TRPV4 channels disruption. Nanotoxicology 2022a ; 16 : 29. #equal contribution as last authors.
- Germande O, Ducret T, Quignard J-F, Deweirdt J, Freund-Michel V, Errera MH, Cardouat G, Vacher P, Muller B, Berger P, Guibert C, Baudrimont M#, Baudrimont I#. NiONP-Induced Oxidative Stress and Mitochondrial Impairment in an In Vitro Pulmonary Vascular Cell Model Mimicking Endothelial Dysfunction. Antioxidants 2022b ; 11 : 847. #equal contribution as last authors.
- Barbeau S, Gilbert G, Cardouat G, Baudrimont I, Freund-Michel V, Guibert C, Marthan R, Vacher P, Quignard J-F, Ducret T. Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules 2021 ; 11 : 1389.
- Chappe Y, Michel P, Joushomme A, Barbeau S, Pierredon S, Baron L, Garenne A, Poulletier De Gannes F Hurtier A, Mayer S, Lagroye I, Quignard J-F, Ducret T, Compan V, Franchet C, Percherancier Y. High-throughput screening of TRPV1 ligands in the light of the Bioluminescence Resonance Energy Transfer technique. Molecular Pharmacology 2021 ; 100 : 237.
- Crobeddu B, Baudrimont I, Deweirdt J, Sciare J, Badel A, Camproux AC, Bui LC, Baeza-Squiban A. Lung Antioxidant Depletion: A Predictive Indicator of Cellular Stress Induced by Ambient Fine Particles. Environmental science and technology 2020 ; 54 : 2360.
- Deweirdt J, Quignard J-F, Lacomme S, Gontier E, Mornet S, Savineau J-P, Marthan R, Guibert C, Baudrimont I. In vitro study of carbon black nanoparticles on human pulmonary artery endothelial cells: effects on calcium signaling and mitochondrial alterations. Archives of toxicology 2020 ; 94 : 2331.
- Freund-Michel V, Cardoso Dos Santos M, Guignabert C, Montani D, Phan C, Coste F, Tu L, Dubois M, Girerd B, Courtois A, Humbert M, Savineau JP, Marthan R, Muller B. Role of Nerve Growth Factor in Development and Persistence of Experimental Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine 2015 ; 192 : 342.
