Coordinators : Christelle Guibert (DR INSERM), Thomas Ducret (PU), Jean-François Quignard (PU), Guillaume Cardouat (MCF), Eric Dumas De La Roque (PH), Claire-Marie Pilard (PH)
Participants : Solène Barbeau (post-doctorante), Laure Gassiat (doctorante), Frédéric Coatleven (PH), Sophie Martin-Berenguer (PH), Melie Sarreau (PH)
Technical staff : Isabel Gauthereau (IGE),Paul Robillard (TCH), Marilyne Campagnac (TCH), Benjamin Péré (TCH), Alexis Leriche (AI – CDD)
Bronchopulmonary dysplasia (BPD) is the leading respiratory complication in extremely preterm newborns. It results from multiple perinatal insults, including prenatal inflammation, intrauterine growth restriction (IUGR), barotrauma and volotrauma associated with postnatal mechanical ventilation, and oxidative stress related to oxygen therapy.
Histologically, BPD is primarily characterized by arrested alveolar development (hypoalveolarization) and impaired vascular development (hypoangiogenesis), associated with marked inflammation and oxidative stress. BPD affects between 30% and 70% of extremely premature newborns, and 25% to 40% of infants with severe BPD will develop pulmonary hypertension (BPD-PH). BPD-PH is the most severe complication of BPD. Indeed, persistent BPD-PH in the first months of life is associated with a mortality rate of 40% to 50% within two years of diagnosis. Due to progress in neonatal intensive care, survival of premature infants has strongly increased and BPD has become a significant public health concern. Moreover, BPD is a pulmonary disease with long-term consequences into adulthood, including an increased susceptibility to develop pulmonary diseases (e.g., asthma), vascular diseases (e.g., PH), cardiac conditions (e.g., arrhythmias), and neurological disorders (e.g., cognitive impairment).
Currently, no preventive or curative treatment is available for 1) the management of children with or without BPD-associated PH, and 2) BPD survivors, to restore lung function and prevent long-term sequelae.
Improving our understanding of the pathophysiology of this disease is therefore essential to develop targeted and effective therapeutic strategies.
Recent Work
In our laboratory, we have developed a neonatal rat model exposed to chronic hyperoxia (14 days in 90% O₂), which accurately replicates the human pathology of BPD-PH (Figure 1, left) (Dumas de la Roque et al., 2017). To assess the medium- and long-term consequences of BPD-PH, animals are studied at postnatal day 14, day 60 (young adult), and day 180 (adult).
In collaboration with the maternity ward and pathology department of Bordeaux University Hospital (CHU), we also have access to foetal lungs and umbilical cords obtained following medical termination of pregnancy. These tissues allow us to isolate foetal human pulmonary artery smooth muscle cells and vascular endothelial cells.
Using a hyperoxic incubator, we expose these cells to 60% oxygen for 48 hours to investigate the effects of oxygen on these cell types (Figure 1, right).
We have recently demonstrated that celastrol, a natural anti-inflammatory and antioxidant compound derived from traditional Chinese medicine, significantly improves survival in the rat model. Celastrol prevents the development of BPD-PH and may represent a novel therapeutic tool (Pilard et al., 2025).
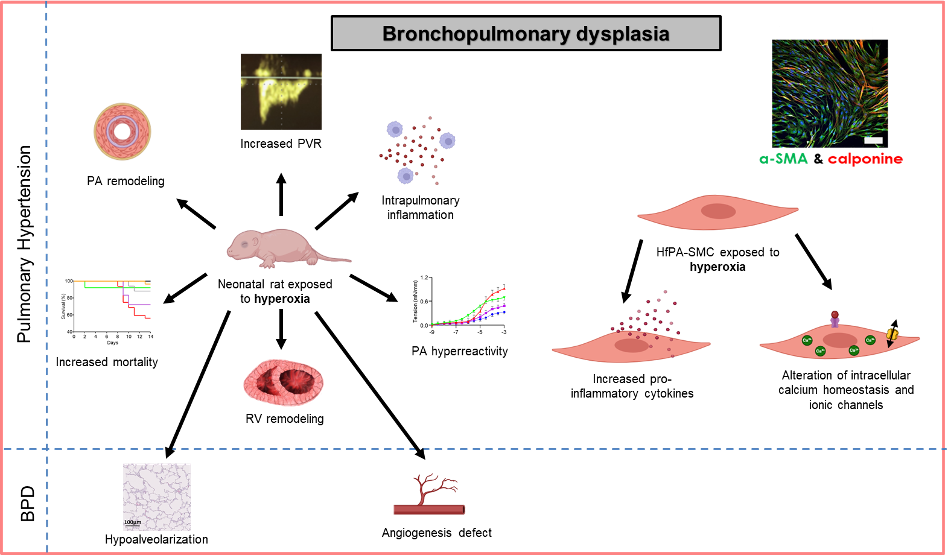
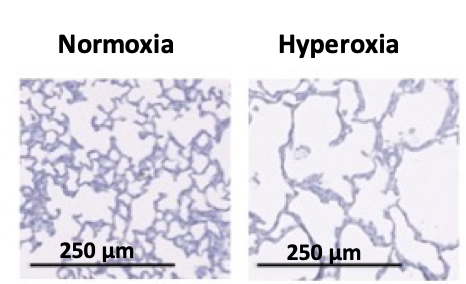
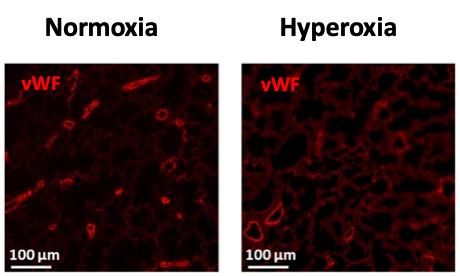
Figure 2: Alveolarization (H&E staining – left) and vascular density (fluorescent labelling of endothelium with von Willebrand factor in red – right) under normoxic and hyperoxic conditions in 14-days-old rat pups.
Ongoing Projects
- Structural and electrophysiological remodeling of the right ventricle
(Coordinator: Claire-Marie Pilard) - Role of osteoprotegerin (OPG)
(Coordinator: Thomas Ducret) - Role of thrombomodulin
(Coordinator: Christelle Guibert) - Role of the GPRX receptor
(Coordinator: Guillaume Cardouat) - Identification of novel natural molecules derived from Brazilian plant biodiversity targeting mechanosensitive ion channels for the treatment of BPD-PH
(Coordinator: Jean-François Quignard)
Collaboration with Prof. Isac Medeiros (Pharmacology Laboratory, Federal University of Paraíba, Brazil)
Funding Sources
- Hemangiol patent royalties – Eric Dumas De La Roque / Christelle Guibert
- Neonatology Research – French Society of Neonatology – Claire-Marie Pilard
- ANR – Endosurf project – Eric Dumas De La Roque
- Prematuration Program – Guillaume Cardouat
- Various grants from the STS Department (incoming mobility, shared equipment) – Jean-François Quignard / Christelle Guibert – Collaboration with Prof. Isac Medeiros (Brazil)
- Medical Research Foundation (FRM) – “FDM – PhD Fellowship for Residents and Assistants” – Claire-Marie Pilard
- “Research, Training, or Evaluation” Program – Chronic Diseases Fund requiring medical-technical support, Bordeaux University Foundation – Christelle Guibert
Main bibliographic references
- Pilard C.M., Cardouat G., Gauthereau I., Gassiat L., DuboisM., Robillard P., Sauvestre F., Pelluard F., Berenguer S., Sarreau M., Claverol S., Tokarski C., Sentilhes L., Coatleven F., Vincienne M., Marthan R., Dumas-de-la-Roque E., Berger P., Friedberg M.K., Renesme L., Freund-Michel V., Guibert C.– * co-derniers auteurs. Celastrol has beneficial effects on pulmonary hypertension associated with bronchopulmonary dysplasia: Preclinical study outcomes Biomedicine and Pharmacotherapy 2025 ; 184 : 117881.
- Dumas-de-la-Roque E., Smeralda G., Quignard JF., Freund-Michel V., Courtois A., Marthan R., Muller B., Guibert C., Dubois M. Altered vasoreactivity in neonatal rats with pulmonary hypertension associated with bronchopulmonary dysplasia: Implication of both eNOS phosphorylation and calcium signaling. PLoS One 2017 ; 12(2) : e0173044.
