Group leader: Gaël Dournes
Group members: Ilyès Ben Lala, Amel Hadj Bouzid
Early diagnosis and individually tailored treatments are the future of medicine. This concept has been designated as ‘personalized medicine’, which aims to deliver the right treatment to the right patient at the right time. However, to reach this objective, we need to find new biomarkers. Biomedical imaging is a key component of personalized medicine through its capacities for earlier diagnoses, for prognostication, for quantification of inter-individual differences that can affect the type and intensity of treatment, for accurate therapy response monitoring, and for guiding innovative and minimally invasive intervention.
One of the major challenges is therefore developing key technologies (imaging, AI, chemistry, instrumentation, etc.) with first clinical outputs, based on local expertise of research teams and existing academic and industrial collaborations to identify the imaging strategies that could have high impact on outcome for the wellbeing of each individual patient.
Current project
Chronic lung diseases represent a major public health problem. Lung imaging enables in vivo evaluation of the disease severity, which is sustained by the recent technological breakthrough in CT and MRI. Lung imaging is poised to play a central role in managing patients with chronic lung diseases, owing to the recent advent of new treatments. Thus, there is a need for novel quantitative imaging biomarkers. Artificial intelligence with deep learning should allow unprecedented possibilities to develop innovative solutions in this contemporary context.
General objectives
- To develop novel deep learning methods, considering the technical and technological particularities of lung imaging using CT and MRI.
- To assess the feasibility of innovative quantitative methods to segment the lung structural and functional abnormalities and predict clinical outcomes such as exacerbation and the response to treatment.
- To assess the generalizability of artificial intelligence-based methods of quantification, from CT to MRI.
- To assess the clinical validity of the developed methods in cohorts of patients suffering from chronic lung diseases.
Main fundings: PHRC-N CFMR Lung; RRI Impact IdEx “Investments for the Future”; TRAIL ANR-10-LABX-57 ; AAPR 2019 IRM pulmonaire 5D allié à l’intelligence artificielle ; AOIs CHU de Bordeaux ; Bourse Fondation du Souffle ; Bourse Broussin-Delorme ; Bourse Alain Rahmouni.
Main references
- Dournes G, Hall CS, Willmering MM, Brody AS, Macey J, Bui S, et al. Artificial intelligence in computed tomography for quantifying lung changes in the era of CFTR modulators. Eur Respir J. 2022; 59(3): 2100844.
- Dournes G, Walkup LL, Benlala I. The clinical use of lung MRI in cystic fibrosis: what, now, how? Chest. 2021; 159.
- Dournes G, Laurent F, Coste F, Dromer C, Blanchard E, Picard F, et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease. Correlation with pulmonary hypertension. Am J Respir Crit Care Med. 2015; 191(1): 63‑70.
- Dournes G, Zysman M, Benlala I, Berger P. [CT imaging of chronic obstructive pulmonary disease: What aspects and what role?]. Rev Mal Respir. 2024; 41(10): 738‑50.
- Bouzid AIH, Baldacci F, De Senneville BD, Hassen WB, Benlala I, Berger P, et al. 3D Semantic Segmentation of Airway Abnormalities on UTE-MRI with Reinforcement Learning on Deep Supervision. In: 2025 IEEE 22nd International Symposium on Biomedical Imaging (ISBI) [Internet]. Houston, TX, USA: IEEE; 2025 [cité 18 mai 2025]. p. 1‑5. Disponible sur: https://ieeexplore.ieee.org/document/10981041/
- Hadj Bouzid AI, Bui S, Benlala I, Berger P, Hutt A, Liberge R, et al. Artificial intelligence-driven volumetric CT outcome score in cystic fibrosis: longitudinal and multicenter validation with/without modulators treatment. Eur Radiol. 2025; 35(2): 815‑27.
- Dournes G, Marthan R, Berger P, Laurent F. Excess Risk of Cancer from Computed Tomography Scan Is Small but Not So Low as to Be Incalculable. Am J Respir Crit Care Med. 2015; 192(11): 1396‑7.
- Dournes G, Grodzki D, Macey J, Girodet PO, Fayon M, Chateil JF, et al. Quiet Submillimeter MR Imaging of the Lung Is Feasible with a PETRA Sequence at 1.5 T. Radiology. 2016; 279(1): 328.
- Dournes G, Menut F, Macey J, Fayon M, Chateil JF, Salel M, et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol. 2016; 26(11): 3811‑20.
- Dournes G, Berger P, Refait J, Macey J, Bui S, Delhaes L, et al. Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis: MR Imaging of Airway Mucus Contrasts as a Tool for Diagnosis. Radiology. 2017; 285(1): 261‑9.
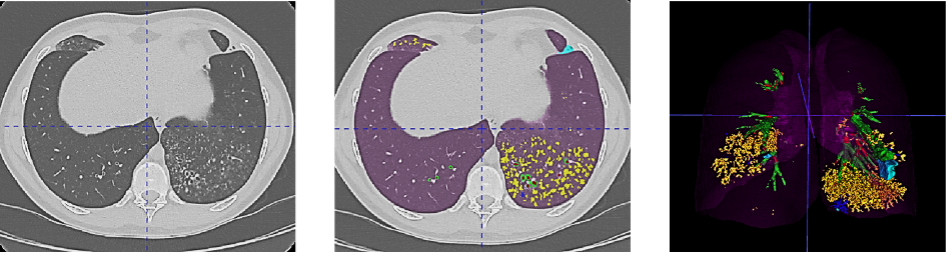
Figure 1. Example of the Normalized Volume of Airway Abnormalities (NOVAA) method. On the left, native CT scan; in the center, AI-automatically labeled CT scan; and on the right, 3D volumetric reconstruction of structural abnormalities. Artificial intelligence enables recognition and semantic segmentation of key structural alterations in airway diseases, such as bronchial dilations (in red), wall thickening (in green), bronchial mucus (in blue), and bronchiolar mucus (in yellow).
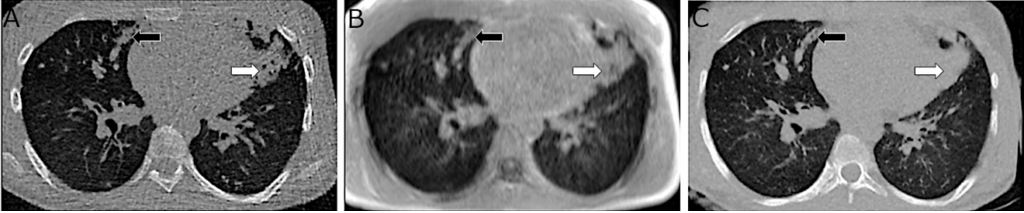
Figure 2. Example of MRI image quality enhancement enabled by AI. On the left, native CT scan; in the center, ultra-short echo time (UTE) MRI; and on the right, synthetic CT scan generated by converting MRI images into a CT-like format. Improved contour delineation, reduced artifacts, and better visibility of structural abnormalities are observed in the synthetic CT scan.