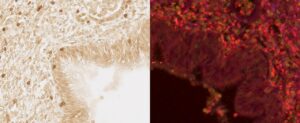
Group leader: Isabelle Dupin
Group members: Marilyne Campagnac, Josquin Courte, Léo Grassion, Pauline Henrot, Eloïse Latouille, Amélie Legrix, Marie-Céline Littré, Elise Maurat, Yamaris Mena Cabrera, Renaud Prevel, Katharina Raasch, Maeva Zysman
Lung exposure to various types of particles, such as those present in cigarette smoke, can lead to chronic obstructive pulmonary disease (COPD). COPD is a common and devastating respiratory disease, characterized by a progressive airflow obstruction. The chronic course of COPD is frequently worsened by acute exacerbations, which contribute considerably to the worldwide increased mortality, morbidity, and health-care costs. Most of these exacerbations are due to lung viral or bacterial infections, causing acute-on-chronic lung inflammation. In addition, around 1/3 of individuals with COPD present skeletal muscle damage, also known as sarcopenia (Henrot et al., 2023a; b), which is itself responsible for increased mortality among patients with COPD.
We hypothesize that alterations in cell-cell and cell-matrix interactions are a major cause of chronic inflammation, fibrotic manifestations and destructive lesions (emphysema) in the lungs and skeletal muscles of patients with COPD. Our group aims at combining basic and translational approaches together with a combination of advanced imaging, innovative cellular and animal models to investigate the interaction between cells and their microenvironment, in the context of COPD and air pollution. Thanks to the close relationship with the University hospital of Bordeaux and physicians from the research group, we have a privileged access to human lung and muscle samples.
Recent achievements
Recently, we have also shown that the CXCL12/CXCR4 axis plays a key role in the pathogenesis of early COPD, supporting further investigation into the use of CXCR4 inhibitors to slow down the progression of COPD (Dupin et al., 2025; Dupin et al., 2019; Henrot et al., 2019). We have oriented our interest towards more physiological tridimensional culture models to investigate mechanisms leading to respiratory disease development (Maurat et al., 2024; Guecamburu et al., 2025) and to test innovative treatments, with the ambition of moving towards personalized medicine for individuals with COPD.
Current projects
1. To investigate the response to particulate pollution and mechanisms of lung resilience using novel organoid model of distal airways and mathematical models (PI: Isabelle Dupin)
Main fundings: ERC CoG KINTSUGI, ANR JCJC BRONCHIOLE

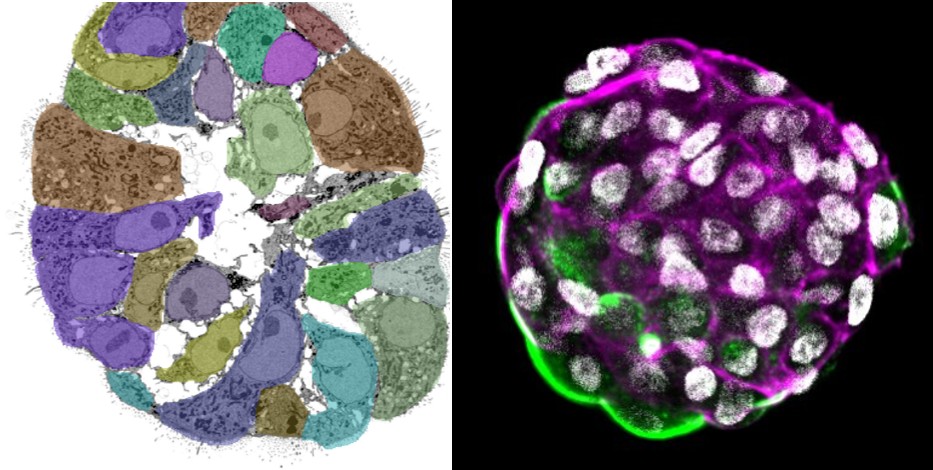
2. To develop a standardized alveolosphere model to better understand emphysema pathophysiology (PI: Maeva Zysman)
Main fundings: ANR PRCE LUMEN, Fondation du Souffle, Chiesi
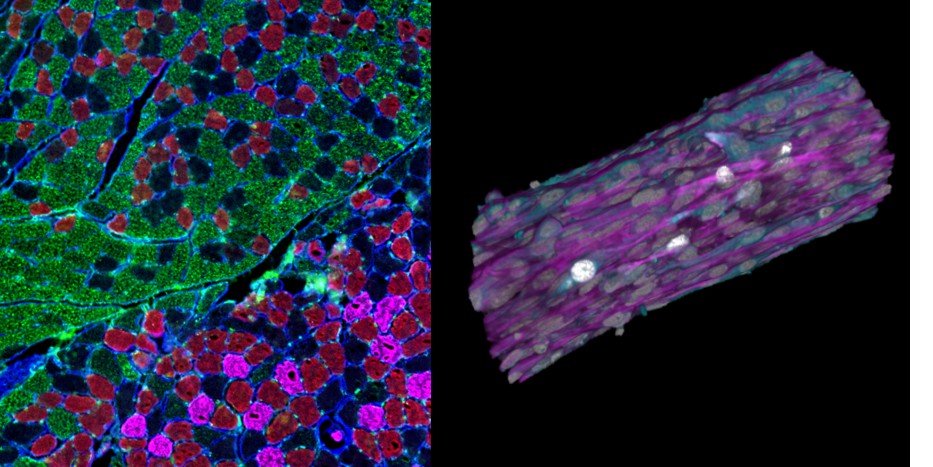
3. To study the role of satellite cells and CXCL12/CXCR4 axis in the development of COPD-associated sarcopenia using animal models and innovative skeletal muscle organoid (PI: Pauline Henrot)
Main fundings: ANR PRC SCOOP, RIE Univ Bordeaux
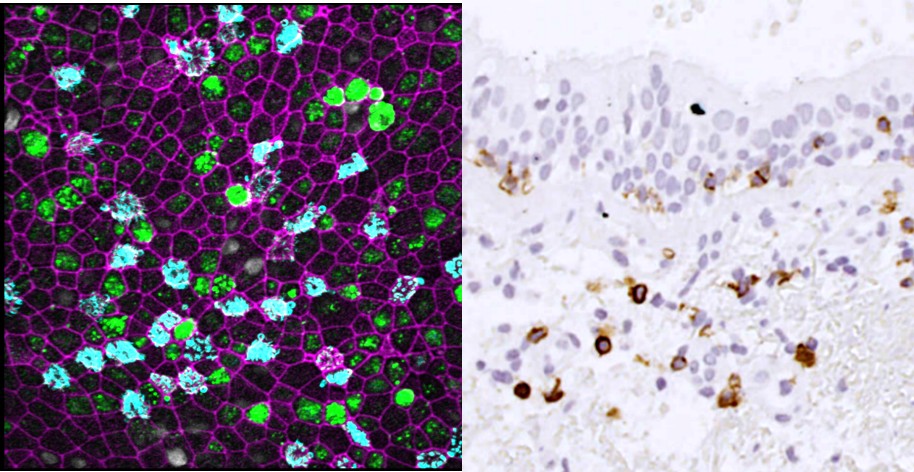
4. To evaluate the efficacy of innovative curative RNA drugs, that could counteract bronchial remodeling and chronic inflammation in COPD (PI: Isabelle Dupin)
Main fundings: ANR PRC LUNATA, AstraZeneca
5. To resume lung epithelium and immune cells interactions to target disease tolerance in respiratory infections using human lung organoid models (PI: Renaud Prével)
Main fundings: collaboration with 4Living Biotech, AOI CHU Bordeaux CHOPIN

Recent publications
- Guecamburu M, Pavot A, Jeannière C, et al. Tunable 3D Alveolosphere Model from Human Alveolar Cells: A Breakthrough Tool to Explore Emphysema Pathophysiology [Internet]. bioRxiv; 2025 [cited 2025 Apr 26]. p. 2025.04.09.645486. Available from: https://www.biorxiv.org/content/10.1101/2025.04.09.645486v1.
- Prevel R, Pernet E, Tran KA, Sadek A, Sadeghi M, Lapshina E, Jurado LF, Kristof AS, Moumni M, Poschmann J, Divangahi M. β-Glucan reprograms alveolar macrophages via neutrophil/IFNγ axis in a murine model of lung injury. Elife. 2025 Jul 8;13:RP102068. doi: 10.7554/eLife.102068. PMID: 40624927; PMCID: PMC12237401.
- Maurat E, Raasch K, Leipold AM, Henrot P, Zysman M, Prevel R, Trian T, Krammer T, Bergeron V, Thumerel M, Nassoy P, Berger P, Saliba AE, Andrique L, Recher G, Dupin I. A novel in vitro tubular model to recapitulate features of distal airways: the bronchioid. Eur Respir J. 2024 Dec 5;64(6):2400562. doi: 10.1183/13993003.00562-2024. PMID: 39231631; PMCID: PMC11627163.
- Dupin I, Henrot P, Maurat E, Abohalaka R, Chaigne S, El Hamrani D, Eyraud E, Prevel R, Esteves P, Campagnac M, Dubreuil M, Cardouat G, Bouchet C, Ousova O, Dupuy JW, Trian T, Thumerel M, Bégueret H, Girodet PO, Marthan R, Zysman M, Freund-Michel V, Berger P. CXCR4 Blockade Alleviates Pulmonary and Cardiac Outcomes in Early Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol. 2025 Oct;73(4):530-544. doi: 10.1165/rcmb.2024-0303OC. PMID: 40198797.
- Raasch K, Henrot P, Hadchouel-Duvergé A, Zysman M, Dupin I. Understanding and manipulating morphogenetic processes to generate in vitro models of airways. Am J Physiol Lung Cell Mol Physiol. 2025 Aug 1;329(2):L234-L254. doi: 10.1152/ajplung.00015.2025. Epub 2025 Jun 27. PMID: 40577330.
- Henrot P, Blervaque L, Dupin I, Zysman M, Esteves P, Gouzi F, Hayot M, Pomiès P, Berger P. Cellular interplay in skeletal muscle regeneration and wasting: insights from animal models. J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):745-757. doi: 10.1002/jcsm.13103. Epub 2023 Feb 21. PMID: 36811134; PMCID: PMC10067506.
- Henrot P, Dupin I, Schilfarth P, Esteves P, Blervaque L, Zysman M, Gouzi F, Hayot M, Pomiès P, Berger P. Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting. Int J Mol Sci. 2023 Mar 29;24(7):6454. doi: 10.3390/ijms24076454. PMID: 37047427; PMCID: PMC10095391.
- Eyraud E, Maurat E, Sac-Epée JM, Henrot P, Zysman M, Esteves P, Trian T, Dupuy JW, Leipold A, Saliba AE, Begueret H, Girodet PO, Thumerel M, Hustache-Castaing R, Marthan R, Levet F, Vallois P, Contin-Bordes C, Berger P, Dupin I. Short-range interactions between fibrocytes and CD8+ T cells in COPD bronchial inflammatory response. Elife. 2023 Jul 26;12:RP85875. doi: 10.7554/eLife.85875. PMID: 37494277; PMCID: PMC10371228.